Thioferrates
|
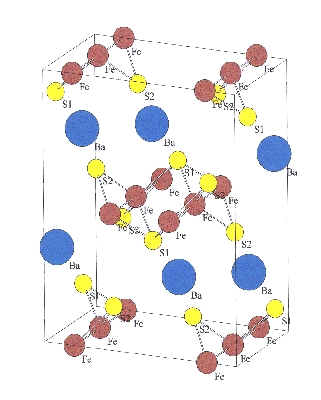
(Ba,K)Fe2S3 Polyanionc infinite [Fe2S3]2- double chains formed by edge-sharing [FeS4] tetrahedron are characteristic for the crystal structure of the thioferrate BaFe2S3 (space group Cmcm). The Ba2+ cations are interspersed between these chains. Crystallographically only one Fe site is present, which should be divalent. Mössbauer investigations reveals, however, an isomer shift pointing towards a mixture of di- and trivalent Fe atoms. For isostructural KFe2S3, with cation charge +1, for Fe a valence state of 2.5 is expected, which may be realized by a random distribution of Fe2+ and Fe3+ at the same crystallographic site. Quite unique for partially ionic compounds, BaFe2S3 and KFe2S3 form a continuous solid solution. This offers the possibility to study the influence of the cation distribution on the not well established character of the bonding between Fe and S within the tetrahedron. BaFe2X, X = S, Se |
Fig.1: Orthorhombic crystal structure
|
Contact: M. Reissner, W. Steiner |